

Contents show Molecular Structure of O3 Let’s first look at the Lewis structure of O3. Due to this, O3 (Ozone) is polar in nature. In O3, the electric dipole moments of the bonds don’t counterbalance one another which results in a net dipole moment. This results in a trigonal planar structure. As both the atoms of Oxygen on sides have the same electronegativity and structure, the double bond keeps on shifting from both and results in resonance. O3 is a polar molecule and it is due to its bent molecular geometry. How is the molecular geometry of O3 determined Here is how its Lewis dot structure looks - Now, the central oxygen has a lone pair and two bond pairs. To satisfy the octet rule, central atom requires to form a double bond on either of its sides with an Oxygen molecule. The central atom has only one lone pair of electrons which is making it stable due to the eight electrons in its outermost orbit. Three electron pairs - (trigonal planar arrangement - AX2 with 2 bonding pairs.
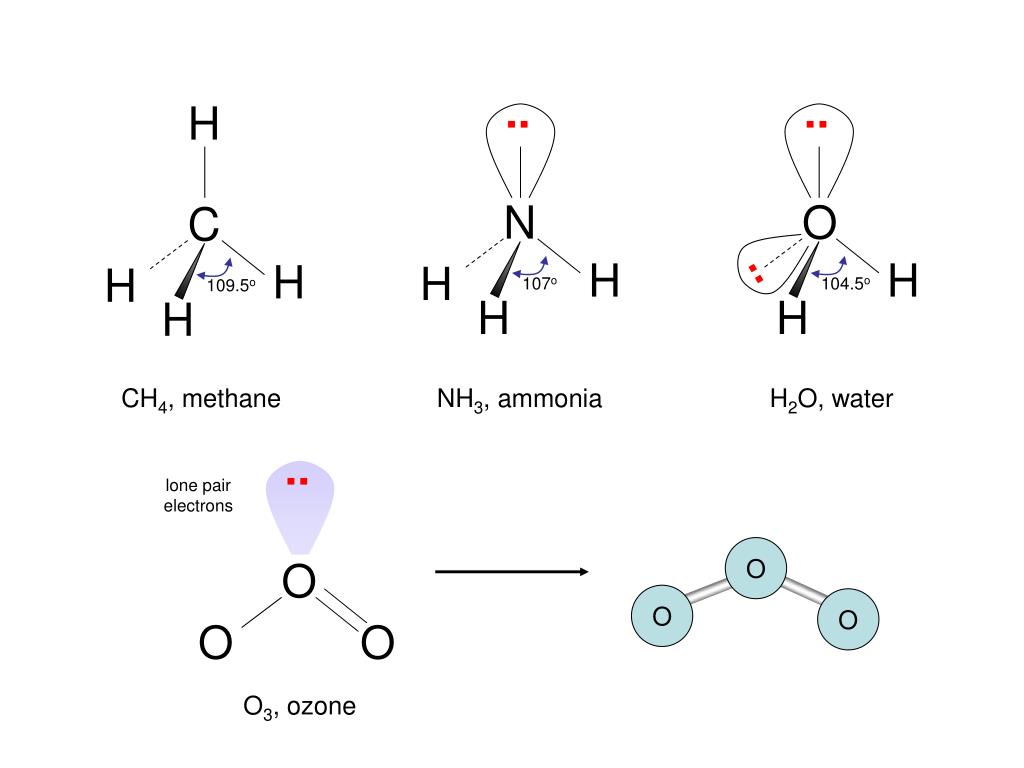
So, one molecule of the Oxygen is in the centre with the other two on the opposite sides of it. As the octet rule applies, the central atom should have eight electrons in its outer shell. However, in order to focus on one aspect of ozones structure, we will use a hybrid approximation. Hello GuysToday in this video we are going to share a detailed yet simple method to determine the molecular geometry of Ozone molecules. There are six valence electrons for each molecule of Oxygen in ozone and thus the total number of valence electrons is \. Ozone is a fairly simple molecule, with only three atoms. Ozone has all three oxygen atoms and it’s a dihedral molecule with \. Ozone O3 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards. (c) In the SO2 molecule, both of the bonds between. Ozone O3 is generated through the passage of oxygen O2 through a high. It has three lone pairs to form pi bonds with other atoms. One point is earned for the molecular geometry consistent with the Lewis diagram in part (a). Ozone is an allotropic molecular form of oxygen containing three atoms of oxygen (O3). That is why it can form two sigma bonds with the other atoms.


 0 kommentar(er)
0 kommentar(er)
